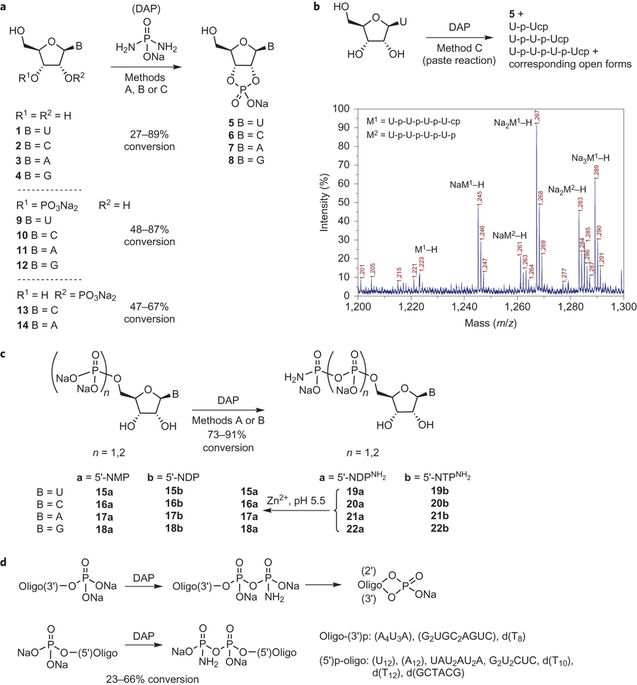
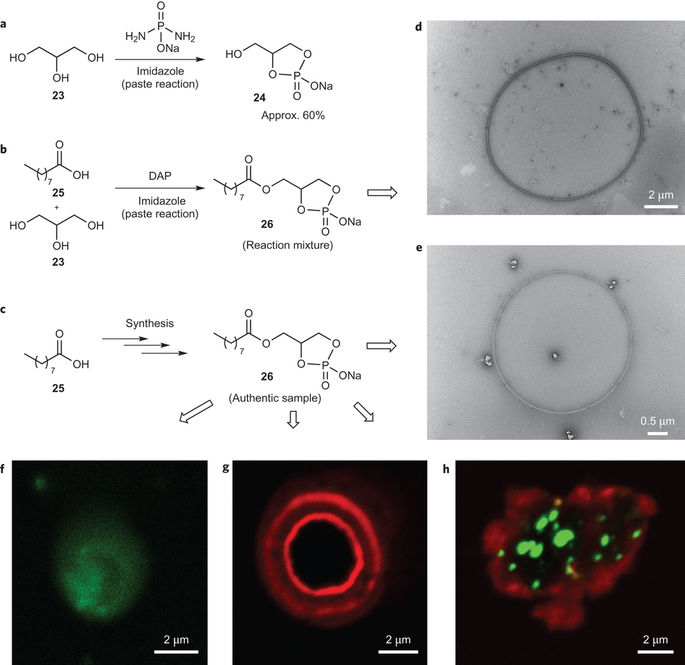
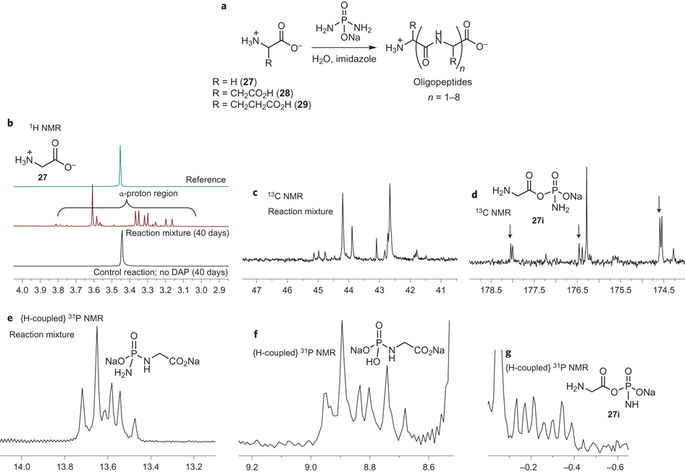
Abstract
Prebiotic phosphorylation of (pre)biological substrates under aqueous conditions is a critical step in the origins of life. Previous investigations have had limited success and/or require unique environments that are incompatible with subsequent generation of the corresponding oligomers or higher-order structures. Here, we demonstrate that diamidophosphate (DAP)—a plausible prebiotic agent produced from trimetaphosphate—efficiently (amido)phosphorylates a wide variety of (pre)biological building blocks (nucleosides/tides, amino acids and lipid precursors) under aqueous (solution/paste) conditions, without the need for a condensing agent. Significantly, higher-order structures (oligonucleotides, peptides and liposomes) are formed under the same phosphorylation reaction conditions. This plausible prebiotic phosphorylation process under similar reaction conditions could enable the systems chemistry of the three classes of (pre)biologically relevant molecules and their oligomers, in a single-pot aqueous environment.
References
- 1.
Lohrmann, R. & Orgel, L. E. Prebiotic synthesis: phosphorylation in aqueous solution. Science 161, 64–66 (1968).
- 2.
Schwartz, A. W. Specific phosphorylation of the 2′- and 3′-positions in ribonucleosides. J. Chem. Soc. D 1393a (1969).
- 3.
Leman, L. J., Orgel, L. E. & Ghadiri, M. R. Amino acid dependent formation of phosphate anhydrides in water mediated by carbonyl sulfide. J. Am. Chem. Soc. 128, 20–21 (2006).
- 4.
Powner, M. W., Gerland, B. & Sutherland, J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009).
- 5.
Burcar, B. et al. Darwin's warm little pond: a one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. 55, 13249–13253 (2016).
- 6.
Kim, H. J. et al. Evaporite borate-containing mineral ensembles make phosphate available and regiospecifically phosphorylate ribonucleosides: borate as a multifaceted problem solver in prebiotic chemistry. Angew. Chem. Int. Ed. 55, 15816–15820 (2016).
- 7.
Pasek, M. A. & Kee, T. P. in Origins of Life: The Primal Self-Organization (eds Egel, R., Lankenau, D.-H. & Mulkidjanian, A. Y.) 57–84 (Springer, 2011).
- 8.
Gull, M. Prebiotic phosphorylation reactions on the early Earth. Challenges 5, 193–212 (2014).
- 9.
Ruiz-Mirazo, K., Briones, C. & de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366 (2014).
- 10.
Krishnamurthy, R., Guntha, S. & Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 39, 2281–2285 (2000).
- 11.
Coggins, A. J. & Powner, M. W. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 9, 310–317 (2017).
- 12.
Meznik, L., Thomas, B. & Töpelmann, W. Zum Reaktionsverhalten von phosphoroxidtriamid in alkalischen lösungen. Zeit. Chemie 22, 211–211 (1982).
- 13.
Richter, S., Töpelmann, W. & Lehmann, H.-A. Zur hydrolyse der phosphorsäureamide. Zeit. Anorg. Allgem. Chemie 424, 133–143 (1976).
- 14.
Bishop, M. J., Lohrmann, R. & Orgel, L. E. Prebiotic phosphorylation of thymidine at 65 °C in simulated desert conditions. Nature 237, 162–164 (1972).
- 15.
Lohrmann, R. & Orgel, L. E. Urea–inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 171, 490–494 (1971).
- 16.
Schoffstall, A. M. Prebiotic phosphorylation of nucleosides in formamide. Origins Life Evol. Biosph. 7, 399–412 (1976).
- 17.
Cafferty, B. J., Fialho, D. M., Khanam, J., Krishnamurthy, R. & Hud, N. V. Spontaneous formation and base pairing of plausible prebiotic nucleotides in water. Nat. Commun. 7, 11328 (2016).
- 18.
Bowler, F. R. et al. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat. Chem. 5, 383–389 (2013).
- 19.
Kervio, E., Sosson, M. & Richert, C. The effect of leaving groups on binding and reactivity in enzyme-free copying of DNA and RNA. Nucleic Acids Res. 44, 5504–5514 (2016).
- 20.
Apel, C. L., Deamer, D. W. & Mautner, M. N. Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim. Biophys. Acta 1559, 1–9 (2002).
- 21.
Gendaszewska-Darmach, E. & Drzazga, A. Biological relevance of lysophospholipids and green solutions for their synthesis. Curr. Org. Chem. 18, 2928–2949 (2014).
- 22.
Dubois, M. & Zemb, Th. Swelling limits for bilayer microstructures: the implosion of lamellar structure versus ordered lamellae. Curr. Opin. Colloid Interface Sci. 5, 27–37 (2000).
- 23.
Zhu, T. F. & Szostak, J. W. Exploding vesicles. J. Sys. Chem. 2, 4 (2011).
- 24.
Lawless, J. G. & Yuen, G. U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282, 396–398 (1979).
- 25.
Szostak, J. W. An optimal degree of physical and chemical heterogeneity for the origin of life? Phil. Trans. R. Soc. Lond. B 366, 2894–2901 (2011).
- 26.
Dhiman, R. S., Opinska, L. G. & Kluger, R. Biomimetic peptide bond formation in water with aminoacyl phosphate esters. Org. Biomol. Chem. 9, 5645–5647 (2011).
- 27.
Knowles, J. Enzyme-catalyzed phosphoryl transfer reactions. Ann. Rev. Biochem. 49, 877–919 (1980).
- 28.
Lassila, J. K., Zalatan, J. G. & Herschlag, D. Biological phosphoryl-transfer reactions: understanding mechanism and catalysis. Ann. Rev. Biochem. 80, 669–702 (2011).
- 29.
Frederix, P. W. J. M. et al. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels. Nat. Chem. 7, 30–37 (2015).
- 30.
Griesser, H. et al. Ribonucleotides and RNA promote peptide chain growth. Angew. Chem. Int. Ed. 56, 1219–1223 (2017).
- 31.
Adamala, K. & Szostak, J. W. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 5, 495–501 (2013).
- 32.
Karki, M., Gibard, C., Bhowmik, S. & Krishnamurthy, R. Nitrogenous derivatives of phosphorus and the origins of life: plausible prebiotic phosphorylating agents in water. Life (Basel). 7, E32 (2017).
- 33.
Lazcano, A. Complexity, self-organization and the origin of life: the happy liaison? Origins of Life 2009, 13–22 (2009).
- 34.
Eschenmoser, A. The TNA-family of nucleic acid systems: properties and prospects. Orig. Life Evol. Biosph. 34, 277–306 (2004).
- 35.
Anastasi, C. et al. The search for a potentially prebiotic synthesis of nucleotides via arabinose-3-phosphate and its cyanamide derivative. Chem. Eur. J. 14, 2375–2388 (2008).
- 36.
Yamagata, Y., Watanabe, H., Saitoh, M. & Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 352, 516–519 (1991).
- 37.
Feldmann, W. & Thilo, E. Zur chemie der kondensierten Phosphate und Arsenate. XXXVIII. Amidotriphosphat. Zeit. Anorg. Allgem. Chemie. 328, 113–126 (1964).
- 38.
Thilo, E. Zur strukturchemie der kondensierten anorganischen Phosphate. Angew. Chem. 77, 1056–1066 (1965).
- 39.
Pasek, M. A. & Lauretta, D. S. Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 5, 515–535 (2005).
- 40.
Bryant, D. E. & Kee, T. P. Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem. Commun. 2006, 2344–2346 (2006).
- 41.
Turner, B. E. & Bally, J. Detection of interstellar PN: the first identified phosphorus compound in the interstellar medium. Astrophys. J. 321, L75–L79 (1987).
- 42.
Ziurys, L. M. Detection of interstellar PN: the first phosphorous-bearing species observed in molecular clouds. Astrophys. J. 321, L81–L85 (1987).
- 43.
Rivilla, V. M. et al. The first detections of the key prebiotic molecule PO in star-forming regions. Astrophys. J. 826, 161 (2016).
- 44.
Oró, J., Miller, S. L. & Lazcano, A. The origin and early evolution of life on Earth. Annu. Rev. Earth Planetary Sci. 18, 317–356 (1990).
Acknowledgements
The work was supported by a grant from the Simons Foundation to R.K. (327124) and NASA (NNX14AP59G). This is manuscript #29523 from The Scripps Research Institute. The authors thank M. Wood, T. Fassel and W.B. Kiosses of the Core Microscope Facility of TSRI, M. Janssen for initial TEM measurements, J. Kelly's laboratory for DLS measurements, L. Leman for help with analysis of peptides and the S.F. Dowdy laboratory for MALDI-TOF analysis. The authors also thank R. Ghadiri, D. Deamer, S. Mansy, P. Banerjee, J. Szostak, G. Joyce and our lab members for discussions.
Author information
Author notes
- Clémentine Gibard
- , Subhendu Bhowmik
- & Megha Karki
These authors contributed equally to this work.
Affiliations
Department of Chemistry, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA
- Clémentine Gibard
- , Subhendu Bhowmik
- , Megha Karki
- , Eun-Kyong Kim
- & Ramanarayanan Krishnamurthy
Authors
Search for Clémentine Gibard in:
Search for Subhendu Bhowmik in:
Search for Megha Karki in:
Search for Eun-Kyong Kim in:
Search for Ramanarayanan Krishnamurthy in:
Contributions
R.K. conceived the project. R.K., C.G., S.B., M.K. and E.-K.K. designed the experiments. C.G. and S.B. performed the nucleoside/nucleotide/oligonucleotide phosphorylation experiments. M.K., S.B. and C.G. performed the amino acid phosphorylation experiments. M.K. performed the liposome studies. E.-K.K. and S.B. made the initial observations of DAP-mediated phosphorylation. R.K. wrote the paper with input from C.G., S.B., M.K. and E.-K.K. All authors discussed the results and commented on the manuscript. C.G., S.B. and M.K. contributed equally to this work.
Competing interests
The authors declare no competing financial interests.
Corresponding author
Correspondence to Ramanarayanan Krishnamurthy.
Supplementary information
PDF files
- 1.
Supplementary information
Supplementary information