‘Rewired’ mice show signs of longer lives with fewer age-related illnesses.
While developing a new cancer drug, researchers at The Wistar Institute discovered that mice lacking a specific protein live longer lives with fewer age-related illnesses. The mice, which lack the TRAP-1 protein, demonstrated less age-related tissue degeneration, obesity, and spontaneous tumour formation when compared to normal mice. Their findings could change how scientists view the metabolic networks within cells.
In healthy cells, TRAP-1 is an important regulator of metabolism and has been shown to regulate energy production in mitochondria, organelles that generate chemically useful energy for the cell. In the mitochondria of cancer cells, TRAP-1 is universally overproduced. The study, which appears in the journal Cell Reports, shows how ‘knockout’ mice bred to lack the TRAP-1 protein compensate for this loss by switching to alternative cellular mechanisms for making energy.
The researchers observed an astounding change in TRAP-1 knockout mice, where they show fewer signs of aging and are less likely to develop cancers. The findings provide an unexpected explanation for how TRAP-1 and related proteins regulate metabolism within our cells. Researchers usually link the reprogramming of metabolic pathways with human diseases, such as cancer. What the team didn’t expect to see were healthier mice with fewer tumours.
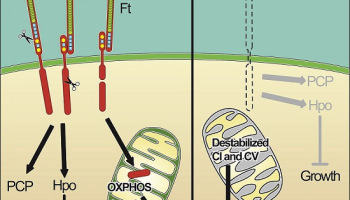
The group created the TRAP-1 knockout mice as part of their ongoing investigation into their novel drug, Gamitrinib, which targets the protein in the mitochondria of tumour cells. TRAP-1 is a member of the heat shock protein 90 (HSP90) family, which are ‘chaperone’ proteins that guide the physical formation of other proteins and serve a regulatory function within mitochondria. Tumours use HSP90 proteins, like TRAP-1, to help survive therapeutic attack. In tumours, the loss of TRAP-1 is devastating, triggering a host of catastrophic defects, including metabolic problems that ultimately result in the death of the tumour cells. Mice that lack TRAP-1 from the start, however, have three weeks in the womb to compensate for the loss of the protein.
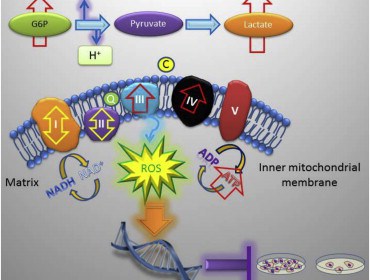
The researchers found that in their knockout mice, the loss of TRAP-1 causes mitochondrial proteins to misfold, which then triggers a compensatory response that causes cells to consume more oxygen and metabolize more sugar. This causes mitochondria in knockout mice to produce deregulated levels of ATP, the chemical used as an energy source to power all the everyday molecular reactions that allow a cell to function.
This increased mitochondrial activity actually creates a moderate boost in oxidative stress (‘free radical damage’) and the associated DNA damage. While DNA damage may seem counterproductive to longevity and good health, the low level of DNA damage actually reduces cell proliferation, slowing growth down to allow the cell’s natural repair mechanisms to take effect.
According to the team, their observations provide a mechanistic foundation for the role of chaperone molecules, like HSP90, in the regulation of bioenergetics in mitochondria, how cells produce and use the chemical energy they need to survive and grow. Their results explain some contradictory findings in the scientific literature regarding the regulation of bioenergetics and dramatically show how compensatory mechanisms can arise when these chaperone molecules are taken out of the equation.
The findings strengthen the case for targeting HSP90 in tumour cells, but they also open up a fascinating array of questions that may have implications for metabolism and longevity. The team predict that the TRAP-1 knockout mouse will be a valuable tool for answering these questions.
Source: The Wistar Institute
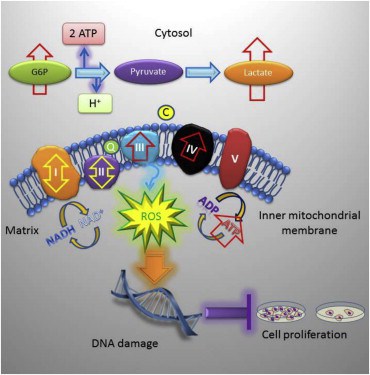
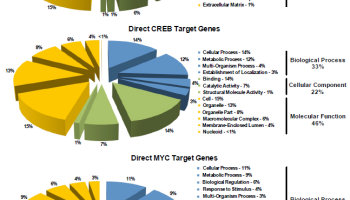
TRAP-1 knockout mice have reduced age-associated pathologies. Loss of TRAP-1 upregulates oxidative phosphorylation and glycolysis transcriptomes. TRAP-1-deleted cells have deregulated mitochondrial respiration and enhanced glycolysis. TRAP-1 deletion induces oxidative stress, DNA damage, and reduced cell proliferation. Deletion of the Mitochondrial Chaperone TRAP-1 Uncovers Global Reprogramming of Metabolic Networks. Altieri et al 2014.